30 out The importance of thorough clinical and histopathologic evaluation in the proper diagnosis and treatment of benign and malignant lesions
Carlos Eduardo Xavier dos Santos Ribeiro da Silva, DDS ; Artur Cerri, DDS; Francisco Octávio Pacca, DDS; Luc Louis Maurice Weckx,DDS; Ines Velez, DDS, MS; Michael A. Siegel, DDS, MS
Learning Objectives
After reading this article, the reader should be able to:
* Describe ameloblastomas and the differences among their classifications.
* Discuss the variety of benign and malignant palatal lesions.
* Describe characteristics of how oral lesions – benign or malignant – may present upon intraoral examination.
* Explain the importance of obtaining histopathologic evaluations of lesion tissue removed from the oral cavity to render accurate diagnoses.
Abstract
Rendering a diagnosis of benign or malignant lesions in the oral environment based solely on clinical and radiographic examination is insufficient to ensure the delivery of proper treatment and the realization of the best long-term prognosis. Because many lesions exhibit characteristics similar to those of either less-aggressive or more destructive pathologies, clinicians must understand the anatomy of the tissues inherent to each area of the oral cavity, as well as the pathologies of different lesions. Additionally, when suspicious lesions are encountered, histopathologic examination should be part of the diagnostic process. This article describes several classifications of benign and malignant lesions found in the oral cavity and presents three cases to demonstrate the manner in which thorough intraoral, head/neck cancer, and histopathologic evaluations were used for diagnosis and treatment planning.
Lesson
Throughout the course of practice, clinicians may encounter patients who present with lesions in the oral cavity. Such lesions may affect the hard or soft tissues – and be either benign or malignant. Correctly diagnosing oral lesions based on clinical and radiographic examination alone may be challenging because their appearance and characteristics initially may be indicative of a different pathology.
Understanding the lesions of the jaw and the hard or soft palate requires appreciation of the different tissues inherent to each area. Knowledge of the complexity of the salivary glands and their structural components is also necessary.
During a vigilant intraoral examination, a high level of knowledge-based suspicion, as well as conducting a thorough head and neck cancer examination, are important to establishing an early and correct diagnosis of the lesion and ensuring the best prognosis for the patient. Additionally, however, microscopic histopathologic evaluation of all tissue removed from the oral cavity is paramount to determining appropriate treatment protocol, particularly when clinical and radiographic findings may suggest another pathology and, therefore, therapy for the lesion in question.
This article describes several classifications of lesions found in the oral cavity (hard/soft palate and jaws) and their respective characteristics. To emphasize the need for thorough intraoral and histopathologic evaluations to ensure the best prognosis for the patient, three cases involving benign and malignant lesions are presented. The process engaged in each case for rendering a differential and definitive diagnosis is also discussed, in addition to any recommended treatment.
Ameloblastoma
The ameloblastoma is a benign, locally aggressive, and invasive neoplasm exclusively affecting the mandible that frequently recurs after treatment.1 Guzak first described it in 1826, and Falkson provided a histological description in 1879.2
Ameloblastoma is derived from odontogenic epithelium, but there are some doubts about its histogenesis. Theories to explain the histogenesis of ameloblastomas postulate that lesions may arise from epithelial remnants of the enamel organ, proliferation of the epithelium that encases the odontogenic cysts, or the cells of the oral mucosa.3,4
According to The World Health Organization, ameloblastomas are classified as conventional, unicystic, and peripheral. Histologic subtypes are desmoplastic, follicular, granular cell, plexiform, and acanthomatous.5,6 Although locally invasive but benign, two related entities can metastasize: the histologically benign ameloblastoma that exhibits malignant behavior (ie, malignant ameloblastoma) and the ameloblastoma with malignant microscopic manifestations (ie, ameloblastic carcinoma).7
The conventional ameloblastoma demonstrates asymptomatic pathology, evolves slowly, and occurs at any age. The most frequent multicystic variant presents in patients anywhere in their 30s through their 70s; the unicystic ameloblastoma is more common in patients who are in their 20s.8
The occurrence of ameloblastoma is independent of sex or ethnic origin, and approximately 80% of cases affect the posterior mandible and ascending ramus.9 It is also commonly associated with an unerupted third molar.
Radiographically, ameloblastomas manifest as osteolytic, well-defined, multilocular lesions showing bone septa, contributing to their soap bubble or honeycomb appearance. Early lesions are unilocular. Root resorption of the involved teeth frequently occurs.10,11
Although ameloblastoma is a benign neoplasm, clinicians have sought suitable treatments because of its typically local, aggressive behavior. Extensive resections have been chosen for solid and multicystic ameloblastomas, whereas those that are unicystic have been treated with enucleation. However, there is a tendency among oral surgeons to adopt less aggressive procedures because the relapse ratios of the lesion are not very different when treated with conservative therapies (eg, enucleation and cryosurgery) compared to radical methods (eg, segmental resection with or without bone reconstruction).
Curi et al advocate that when choosing treatments for ameloblastoma, the patient’s morbidity and quality of life must be considered because extensive resections provoke such problems as chewing difficulties, mutilation, facial deformity, and abnormal mandibular movements.12 Additionally, these authors note that after observing 46 cases of solid ameloblastomas that were treated with surgical enucleation followed by cryosurgery, clinical and radiographic follow-up of the surgical complications showed that – in 30.6% of the cases – there was a local recurrence. Complications included wound dehiscence, paresthesia, infection, and pathologic fracture.12
Feinberg and Steinberg13, along with Neville14, claimed that the recurrence ratios for solid ameloblastomas were between 15% and 25% whenever more radical treatment measures were used. Nakamura et al. described a conservative therapy for cystic ameloblastomas by marsupialization and posterior enucleation that was efficient and resulted in few relapses.15 Radiotherapy, as an auxiliary therapy for ameloblastoma, was recommended in the past but then abandoned because – in addition to the risk of bone necrosis and malignant transformation caused by the radiation – the lesion is resistant to radiotherapy.16
Palatal Lesions
Torus palatinus, the most common palatal entity, presents as an osseous, exophytic, centrally located, midline, and usually symmetric asymptomatic lesion of the hard palate. The epithelial tissues covering tori are attenuated (ie, stretched) because of the underlying bony prominence, subjecting this site to ulcerations of traumatic origin. Inflammatory lesions of dental origin are also common in the hard palate.14
Malignant Squamous Epithelial Neoplasms
Malignant squamous epithelial neoplasms – such as squamous cell carcinoma, verrucous carcinoma, and carcinoma of the maxillary sinus – may also appear in the hard palate. Carcinoma of the maxillary sinus usually remains asymptomatic for long periods of time. Eventually, the tumor grows to fill the sinus and diagnosis is rendered when the lesion has produced a bulge of the palatal or alveolar ridge area. This tumor usually is associated with elderly patients.14
Squamous cell carcinoma of the soft palate is often painful, may cause dysphagia, and offers a worse prognosis than tumors located in more anterior locations. Squamous cell carcinomas extending from the maxillary alveolar ridge onto the hard palate may present a diagnostic challenge because they mimic periodontal disease or pyogenic granulomata. Alveolar and palatal carcinomas are usually painless. Verrucous carcinoma is a type of squamous cell carcinoma that exhibits a papillary, white clinical appearance; behaves indolently; and rarely metastasizes. The most common locations for this lesion are the hard palate and the alveolar ridge; it is often associated with elderly patients wearing complete denture prostheses.14
Salivary Gland Tumors
A wide range of conditions can develop in the numerous small minor salivary glands located within the submucosa of the palate. For example, necrotizing sialometaplasia is a salivary gland condition that usually appears as a crater-like defect of the posterior palate, may be associated with pregnancy, and while ominous in appearance, will usually resolve spontaneously following biopsy confirmation of the diagnosis.14
Salivary gland tumors represent a diverse group of neoplasms; the majority are of ductal or acinic epithelial derivation. Although tumors of salivary glands are not common, they are not rare in occurrence. The biologic behavior of salivary gland tumors is paradoxical. A benign tumor of salivary glands tends to exhibit a more aggressive behavior pattern than the usual benign tumor; a malignant tumor of salivary glands is less aggressive than the usual malignant tumor.17
Minor salivary gland tumors have an affinity for the posterior hard palate and the soft palate and virtually never develop in the midline; this is probably due to the natural distribution of salivary tissues throughout the palate. Both benign and malignant salivary gland neoplasms of the palate appear as well-circumscribed, dome-shaped and smooth-surfaced, nonmovable swellings that exhibit a very slow growth pattern. Pain and ulceration are occasionally seen in adenocarcinomas at this site.17
The most common benign salivary gland tumor of the palate is the pleomorphic adenoma. The most common malignant tumors of the palate are adenoid cystic carcinoma (ie, cylindroma), polymorphous low-grade adenocarcinoma, and mucoepidermoid carcinoma. Most of these tumors, both benign and malignant, are asymptomatic masses or associated with a low level of discomfort. Constant pain in the palate associated with a gradual increase in intensity that is usually present before any noticeable swelling is a common and important characteristic of adenocystic carcinoma. Malignant tumors of the palate may show radiographic evidence of bone destruction and sometimes a radiopacity produced by the neoplastic mass. Intraosseous salivary gland tumors also may develop within the jaws.17
Salivary gland tumors are considered separately from other palatal pathologies because an extensive number of these lesions have been identified. Salivary gland tumors have been classified to include 13 benign tumors and six malignant low-grade, 10 malignant intermediate-grade, and 13 malignant high-grade neoplasms.18
Melanomas
Melanoma is a malignant tumor of melanocytic origin. Although the majority occurs on the skin, they may develop in any site, such as the oral cavity, where melanocytes exist. Significant numbers of melanocytes are normally found in the palatal mucosa. In fact, the most common site for a melanocytic nevus and melanomas of the oral cavity is the palate. Approximately 30% of melanomas develop from previously existing pigmented lesions.14, 19
Oral melanoma appears predominantly on the hard palate or maxillary alveolus and tends to be much more aggressive than its cutaneous counterpart. Early lesions are usually flat and later become nodular and fixed. Most melanomas appear as dark/light brown lesions.14
The prognosis of nonskin melanoma is very poor and primarily dependant on its depth. The “3-D” rule generally can be applied to a melanoma’s prognosis. The darker the melanoma appears clinically, the deeper it is within the epithelium and, therefore, the deadlier the result. It is usually the most aggressive malignant neoplasm of the human body. To clinically differentiate a melanoma from nevus, another pneumonic that can be used is the “ABCD” system, which is widely used by dermatologists: Asymmetry of the lesion; Borders irregularity; Color variation; Diameter greater than 0.6 cm.14
Lymphoma
The posterior palate is also a part of the Waldeyer’s ring and presents numerous aggregates of normal, protective lymphoid tissue that may be the originating site of lymphoma. Lymphoma (ie, lymphosarcoma) encompasses a diverse and complex group of malignancies of lymphoid histogenesis. The most frequent locations of extranodal lymphoma in the head and neck are the posterior hard and soft palate. Lymphomas usually appear as a nontender diffuse mass and are rarely ulcerated. Many salivary gland lymphocytic infiltrates of the palate are actually non-Hodgkin’s B-cell lymphomas of the mucosal associated lymphoid tissue (MALT).14 Sarcomas, malignant tumors of nonepithelial tissue origin, may develop in any location of the human body and usually appear as ulcerated masses in young or middle-aged people.
Case Presentation No. 1: Ameloblastoma
A 25-year-old woman was referred to the Oral Surgery Department of the University of Santo Amaro – UNISA, in São Paulo, Brazil, by the Orthodontics Department for evaluation of a radiolucent lesion in the area of the missing right inferior first molar. During consultation, the patient did not report any relevant information regarding her medical history, family antecedents, dental history, or medication use.
Clinical Examination
Facial symmetry and normal skin texture were observed during the examination of her face. The head and neck examination revealed one inflamed lymph node in the right submandibular area. The remaining lymph nodes were within normal limits.
During the intraoral examination, the patient exhibited expansion of the mandible, as well as normal color of the smooth, brilliant, and well-hydrated mucous membrane. Palpation revealed crepitice of the buccal bone wall, reflecting its thin composition. Radiographically, the lesion in question presented as radiolucent, unilocular, and well-defined by a radiopaque halo. Its diameter measured approximately 2 cm, extending from the root of the mandibular right second molar to the root of the second premolar (Figure 1). The lesion also caused displacement of the right alveolar canal toward the cortical inferior border of the mandible.
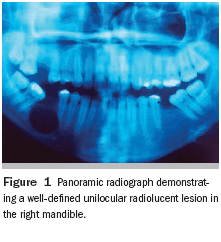
No symptoms were reported, but the lesion was aspirated in order to dismiss the possibility of intraosseous hemangioma. The presence of clear, brownish liquid appeared to confirm a clinical/radiographic diagnosis of a cyst.
Since the lesion radiographically revealed the involvement of the root of the second premolar, vitality testing was performed, with negative results to heat and cold. Therefore, prior to surgery, root canal treatment was performed on this nonvital tooth.
Operative Procedure
Standard surgical preparation procedures were completed with 0.12% chlorhexidine. Using a pterygomandibular technique, the patient was anesthetized on the right side with bupivacaine. An incision was made from the distal portion of the third inferior molar to the mesial region of the canine, followed by blunt gingival retraction. Care was taken to preserve nerve integrity.
After release of the mucoperiosteal flap, the thin and expanded cortical plate was visible and removed with a scalpel. The cavity exhibited a fibrous envelope around its borders and was full of fluid.
Enucleation was carefully completed in one piece to ensure complete removal. Remaining bone tissue showed normal color and consistency, without any clinical signs of pathosis. The flap was then replaced and secured with 4-0 silk sutures.
The patient received routine postsurgical instructions and prescriptions for antibiotics and anti-inflammatory medications. The excised tissue was maintained in 10% formaldehyde and sent to the pathology laboratory for evaluation.
Histopathologic Diagnosis
The histopathologic diagnosis was acanthomatous ameloblastoma with cystic component. Because the clinical/radiographic diagnosis did not agree with the results obtained, the tissue was forwarded to another pathology laboratory, which confirmed the previous laboratory’s results (Figure 2).
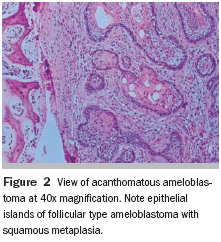
Follow-Up
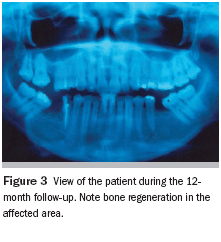
Regular radiographic observation of the patient was required in order to monitor the possibility of lesion relapse, particularly because it originally presented with a well-defined capsule and was removed completely. The patient had been seen for 12 months of follow-ups, with no signs of recurrence. Bone regeneration in the affected area was visible after 12 months (Figure 3).
Case Presentation No. 2: Adenocarcinoma
A 19-year-old man presented to the Nova Southeastern University Department of Oral and Maxillofacial Surgery with a chief complaint of a “painful eruption of the wisdom tooth.” The patient was unaware of any present illness until the acute onset of the “painful eruption” approximately 2 months earlier. Medical history, dental history, social history, and family history were unremarkable. There were no extraoral signs of disease present.
Clinical Examination
Intraoral examination showed an exophytic, crater-form, ulcerated, macerated, and well-circumscribed lesion measuring approximately 2.5 cm in diameter and covered by a fibrinopurulent membrane. The lesion was located palatal to the alveolar ridge and extended to the midline of the palate (Figure 4). No radiographic manifestations were seen in panoramic, periapical, and occlusal radiographs. All teeth in the maxillary left quadrant were vital.
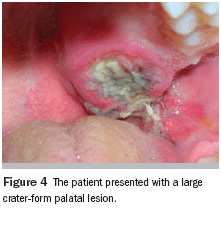
Diagnosis
Palatal torus and odontogenic infection were easily dismissed from the differential diagnosis by reviewing the clinical and radiographic manifestations. This lesion appeared as an ulcerated, fast-growing mass in the area where salivary gland tumors are usually located. Malignant salivary gland tumor was the first clinical consideration because of its location, aggressive nature, and rapid growth rate. Necrotizing sialometaplasia, a crater-like ulcer, does not exhibit the large nodular growth presented in this case. Therefore, it was not considered in the differential diagnosis. Conventional squamous cell carcinoma, verrucous carcinoma, and carcinoma of the maxillary sinus were dismissed because of the patient’s young age.
Sarcomas may be present in young people with the clinical characteristics seen in this case. Approximately 40% of rhabdomyosarcomas and 14% of neurogenic sarcomas occur in the head and neck area.19 They were included in the differential diagnosis.
Liposarcoma, fibrosarcoma, and chondrosarcoma are rare in the oral cavity. Osteosarcoma generally will show radiographic changes. Angiosarcoma exhibits a purple/reddish discoloration of the mucosa.14
Melanoma is commonly a dark colored lesion that usually begins as a brown to black macula with irregular borders, but then a lobulated, nonpainful mass develops later. Satellite lesions are commonly seen. However, amelanotic melanomas (ie, without melanin) occur. About 20% of oral melanomas contain so little pigment that they have a normal mucosal color.14 It is for this reason that melanoma is often included in the differential diagnosis of an aggressive lesion because it has a reputation for mimicking other pathologic entities.
MALT lymphoma was not considered in the differential diagnosis of this case. MALT lymphoma, usually a low-grade non-Hodgkin’s lymphoma, may develop into a high-grade tumor with aggressive behavior. It may appear as a diffuse palatal ulcerated mass, with borders that are not clearly delineated.14
The differential diagnosis in this case included malignant salivary gland tumor, sarcoma, and melanoma.
Operative Procedure
An outpatient incisional biopsy was performed using local anesthesia. Microscopic histopathologic examination revealed infiltrating carcinoma with a uniform pattern and duct and gland-like structures, which was not reminiscent of any other malignant neoplasm of salivary glands. Perineural invasion of the nerve sheath was present.
The diagnosis was adenocarcinoma NOS (ie, not otherwise specified). The patient was treated with a wide surgical excision and adjuvant radiation therapy. The prognosis for this tumor was poor: 56% survival at 5 years.20
Case Presentation No. 3: Working Diagnosis of Melanoma
A 38-year-old man was referred to Nova Southeastern University for the diagnosis of a mass in the left lateral palate. The patient noticed the lesion and a slight swelling of the left side of his face approximately 1 month prior to the consultation. No significant medical, dental, family, or social history was presented.
Clinical Examination
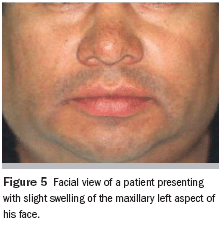
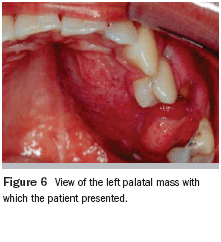
Physical examination revealed that the patient was well developed and well nourished, with a small swelling of the left maxillary aspect of his face (Figure 5). No fever, pain, bleeding, or paresthesias were identified. During the intraoral examination, a large, firm mass covered by normal mucosa that completely occupied the left palate was noted (Figure 6).
The lesion extended from the palatal midline medially into the fornix of the vestibule laterally and covered the alveolar ridge in an edentulous area. The borders of this mass were fixed to the surrounding tissues. The remaining teeth in this quadrant were vital. No radiographic changes were observed in the panoramic, occlusal, or periapical radiographs.
Diagnosis
The differential diagnosis included sarcoma, salivary gland tumor, and MALT. An outpatient incisional biopsy was performed under local anesthesia. The histopathologic examination revealed nests of atypical melanocytic cells with high mitotic activity and pleomorphic nuclei.
A working diagnosis of melanoma was rendered, and the patient was referred for positron emission tomography (PET) studies. After medical consultation, PET scanning, and medical laboratory testing, a definitive diagnosis of primary melanoma of the liver was confirmed. The patient refused treatment and returned to his country of origin.
Discussion
The diagnosis of ameloblastoma in Case No. 1 was possible only as a result of the histolopathologic evaluation that was performed. The clinical and radiographic findings initially suggested the presence of a periapical or residual cyst, when in fact the lesion was more aggressive. Likewise, periapical lesions could also visibly present as expansive and extremely large, mimicking the appearance of different, more destructive lesions. Therefore, Case No.1, in particular, reinforces the importance of histopathologic examination of all tissues removed from a patient, regardless of the initial clinical findings, to establish the correct diagnosis and determine the appropriate follow-up treatment.
Unfortunately, given the patient’s young age, the malignant salivary gland tumor component of the differential diagnosis for Case No. 2 was confirmed through histopathologic examination. Despite the poor prognosis of this tumor, the ability to precisely identify the lesion as soon as possible was paramount to initiating surgical excision and appropriate radiation therapy.
Again, diagnosis and proper treatment of palatal lesions requires a combination of astute clinical observation and histopathologic examination. In Case No. 3, the original differential diagnosis was limited to pathologies of the oral environment. However, histopathologic information suggested the need for further testing, subsequently leading to a diagnosis of primary melanoma of the liver.
Conclusion
The cases presented underscore the importance of routinely performing a thorough intraoral examination, head and neck cancer examination, and histopathologic evaluation of suspicious tissues removed from the oral cavity. Knowledgeable and conscientious clinicians should be able to readily identify lesions requiring attention and treatment. However, the specific characteristics and type of lesion – which can only be confirmed histopathologically – ultimately dictate the most appropriate therapeutic protocol to ensure the best long-term prognosis for the patient.
References
1. Kramer IRH, Pindborg JJ, Shear M. Histological typing of odontogenic tumors. International Histological Classification of Tumours. 2nd ed. Heidelberg: Springer-Verlag; 1992: 11-14.
2. Shafer WG, Hine MK, Levy BM. Tratado de patologia bucal. 4th ed. Rio de Janeiro: Interacmericana; 1985:255-263.
3. Iordanidis S, Makos C, Dimitrakopoulos J, et al. Ameloblastoma of the maxilla. Case report. Aust Dent J. 1999;44(1):51-55.
4. Kawai T, Kishino M, Hiranuma H, et al. A unique case of desmoplastic ameloblastoma of the mandible: a report of a case and brief review of the English language literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(2):258-263.
5. World Health Organization (WHO); 1992.
6. Isacsson G, Andersson L, Forsslund H, et al. Diagnosis and treatment of the unicystic ameloblastoma. Int J Oral Maxillofac Surg. 1986;15(6):759-764.
7. Maia Campos G. Ameloblastoma, a behavioral and histologic paradox (a philosophical approach). Braz Dent J. 1990;1(1):5-15.
8. Kim SG, Jang HS. Ameloblastoma: a clinical, radiographic, and histopathologic analysis of 71 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(6):649-653.
9. Gardner DG. Some current concepts on the pathology of ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82(6):660-669.
10. Regezi JA, Sciubba JJ. Oral Pathology: Clinical-Pathologic Correlations. Philadelphia: Saunders; 1989:554.
11. Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31B(2):86-99.
12. Curi MM, Dib LL, Pinto DS. Management of solid ameloblastoma of the jaws with liquid nitrogen spray cryosurgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(4):339-344.
13. Feinberg SE, Steinberg B. Surgical management of ameloblastoma. Current status of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(4):383-388.
14. Neville, BW, Damm DD, Allen CM, et al. Oral & Maxillofacial Pathology. 2nd ed. Philadelphia: WB Saunders; 2002.
15. Nakamura N, Higuchi Y, Tashiro H, et al. Marsupialization of cystic ameloblastoma: a clinical and histopathologic study of the growth characteristics before and after marsupialization. J Oral Maxillofac Surg. 1995;53(7):748-754, discussion 755-756.
16. Santos JN, Pinto LP, de Figueredo CR, et al. Odontogenic tumors: analysis of 127 cases. Pesqui Odontol Bras. 2001;15(4):308-313.
17. Batsakis JG. Tumors of the Head and Neck: Clinical and Pathological Considerations. 2nd ed. Baltimore: Williams & Wilkins; 1979:1-99.
18. Dardick I. Color Atlas/Text of Salivary Gland Tumor Pathology. New York: Igaku-Shoin Medical Publishers; 1996:33.
19. Pilch BZ. Head and Neck Surgical Pathology. Philadelphia: Lippincott Williams & Wilkins; 2001:389-420.
20. Ellis GL, Auclair PL. Tumors of the Salivary Glands. Washington, DC: Armed Forces Institute of Pathology/Universities Associated for Research and Education in Pathology; 1996:300-73.
Leia Mais
-
Evaluation of bone repair in the mandible of rabbits using biphasic
Leia o artigo completo acessando o PDF abaixo!...
-
Prevalência do genoma do papilomavírus humano no carcinoma espino-celular da língua
Leia o artigo completo acessando o PDF abaixo! ...
